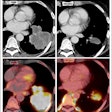
Luminescence imaging technology developer Lightpoint Medical has obtained CE Mark approval for its LightPath imaging system, an intraoperative molecular imaging device.
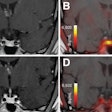
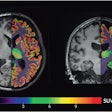
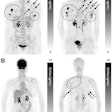
The LightPath system is designed for use in the operating room to help surgeons ensure they have removed all cancerous tissue in a single operation, the firm said. It detects Cerenkov luminescence, a faint light produced by PET imaging agents widely used in cancer diagnosis.
The device is now commercially available in Europe with plans for a U.S. commercial launch in 2016.