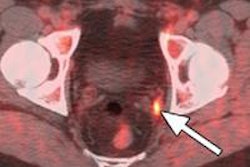
German radiopharmaceutical developer OctreoPharm Sciences has treated the first patients as part of the clinical trials for its SOMscan diagnostic agent.
The patients were treated in a Swiss university hospital during phase I/II clinical trials for SOMscan, which is designed to diagnose neuroendocrine tumors. The trials are expected to be completed by the end of 2014, according to isotope technology developer Eckert & Ziegler. The company made an equity investment in OctreoPharm earlier this year.
SOMscan is based on a carrier molecule that represents a next-generation peptide of antagonistic somatostatin analogs and is being developed for the diagnosis of neuroendocrine tumors. The agent can be used for either diagnosis or therapy, with the molecule labeled with gallium-68 and then with yttrium-90. The European Medicines Agency (EMA) recently awarded SOMscan orphan drug status.