A computer-assisted analysis technique can reliably quantify visceral adipose tissue and serve as a valuable support tool for diagnosing metabolic syndrome, researchers from Colombia and France have concluded.
A team from Universidad de Los Andes in Bogotá, Colombia; Hospital Universitario San Ignacio in Bogotá; and Hôpital Louis Pradel in Lyon, France, found low error rates during both quantitative and qualitative evaluation of their software in quantifying visceral adipose tissue.
"The method significantly reduces user time and effort in visceral adipose tissue quantification, and increases evaluation reliability in human assessments, requiring only minor corrections in most cases," said Dr. Philippe Douek of Hôpital Louis Pradel. He presented the team's experience at RSNA 2012 in Chicago.
Metabolic syndrome is a major health concern for cardiovascular disease and type 2 diabetes. While abdominal perimeter is a predictor for cardiovascular risk, it's difficult to measure in obese patients and also lacks predictive accuracy. Real cardiovascular risk is directly linked to the amount of visceral adiposity, but only a limited number of tools have been developed to date to assist in visceral adipose tissue measurement, according to Douek.
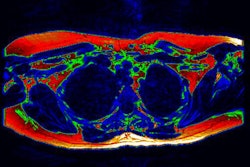
As a result, the multi-institutional team sought to develop a fast and reliable method for segmenting and quantifying visceral adipose tissue on CT images. Their software, called Computer Assisted Analysis of Visceral Adipose Tissue (CAAVAT), employs a novel approach that can discriminate between subcutaneous adiposity and visceral adipose tissue, he said.
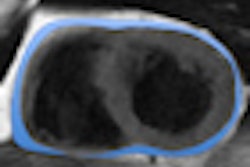
After total adipose tissue is extracted from the images, the software discriminates between visceral and subcutaneous tissues using active contours and a ray-casting approach, according to the researchers. Quantitative measures are then calculated.
To test the system, the researchers applied it on multidetector-row CT abdominal images in 30 patients, using the one CT slice closest to the L3 vertebra. Images were acquired on four different scanners with slice thickness ranging between 5 mm and 10 mm and anisotropic voxels. No special patient preparation was performed.
"So the ideal conditions were not acquired," Douek said.
Two expert radiologists then provided qualitative and quantitative evaluation of the CAAVAT results. Qualitative analysis was performed by visual estimation of the percentage of segmentation error of total adipose tissue (TAT) and visual estimation of the lack of precision in discriminating visceral adipose tissue.
Quantitative analysis was carried out by two trained radiologists, who made corrections to the CAAVAT segmentation results and labeled the corrected pixels using eight error classes. The TAT and visceral adipose tissue areas were measured on both the CAAVAT segmentation and the radiologists' segmentation results.
The mean interobserver estimated percentage of completeness error was 18.8% ± 5.4%, while the mean estimated lack of precision percentage was 13.2% ± 5.2%. At a visceral adipose tissue cutoff point for increased cardiovascular risk (+150 cm2), there was only a difference of less than 10% between the CAAVAT segmentation and the radiologists' segmentation results.
Most of the errors were caused by contents of the intestinal region -- i.e., stool, Douek said.
"Interrater agreement was very high, so this data suggest that CAAVAT-aided segmentation may increase evaluators' accuracy and improve reproducibility," Douek said.
Practical applications of the method include assessments in clinical studies, metabolic syndrome diagnosis and follow-up, and pharmaceutical research, he said.
Douek also noted that results could be improved by using thinner slices at acquisition, employing bowel preparation, and utilizing intravenous contrast.