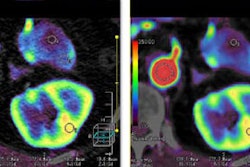
Implantable cardiac device manufacturer Biotronic said it has received CE Mark clearance in Europe for its Pro-MRI technology for implantable defibrillators (ICDs), which permit even whole-body MR scans of patients at 3.0- or 1.5-tesla.
The approval will allow patients to be scanned faster and with high-quality MR imaging when needed, reducing exam time and facilitating workflow, the company said.
Single-chamber and DX implantable defibrillators with atrial diagnostics were already approved with backward compatibility for ultrahigh-field 3-tesla scans with an exclusion zone, the company said. Biotronic ICDs that have already been implanted are now approved for use with MRI scanning free of exclusion zones, Biotronic said, which earned its first approval to implant ICDs in 2011.