The solid component of subsolid pulmonary nodules can be detected automatically -- making it easier to assess the potential malignancy of lung lesions detected on CT, according to researchers from the Netherlands.
Researchers from Nijmegen Medical Center at Radboud University, Nijmegen, Groningen University Medical Center in Groningen, and colleagues from Utrecht and Haarlem, applied software to detect and quantify the solid component of lung nodules automatically in more than 100 nodules and found sensitivities nearing 90%.
"Semiautomatic segmentation of subsolid nodules can differentiate between nonsolid and part-solid nodules with good sensitivity and specificity provided that no vessel segments are included in the segmentation," said Dr. Ernst Scholten in a presentation at ECR 2015.
There is good reason to measure the solid component of subsolid nodules, according to a report that expanded and clarified the Fleischner Society guidelines (Radiology, January 2013, Vol. 266:1, pp. 304-317).
"In the Fleischner Society recommendation for management of subsolid nodules, it is stated that measurement of the size of the solid component of subsolid lesions is important as well as is the percentage of the solid component of that nodules," explained Scholten, who is a researcher with the Radboud University Image Analysis Group. However, a recent paper by Penn et al found significant interreader variability when applying the Fleischner guidelines "as to whether a solid component is present, and even whether the lesion fits the criteria for a solid nodule," he added.
Hoping to find a more objective method of finding and quantifying the solid core than subjective visual evaluation, especially if it could benefit less experienced readers, the group wanted to find out if the solid component of these nodules could be detected with semiautomatic segmentation using a previously validated investigational software program.
First, they sought a reference standard by assigning two radiologists to differentiate 115 sets of nodules into nonsolid and part-solid nodules, with a third radiologist recruited to settle any disagreements; the readers achieved an observer agreement of k = 0.68. Eight sets of nodules were excluded due to inaccurate segmentation, leaving 107 nodules to evaluate.
Testing measurements
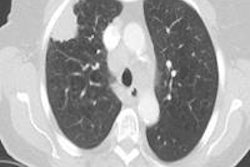
The group applied seven different HU thresholds to determine the best segmentation threshold for detecting the solid component, ranging from -500 HU to -20 HU, and increasing the lower threshold in steps of 50 HU. At the various thresholds, the presence of a solid component was noted along with its size, Scholten said. In one example, showing the lower threshold of -200 HU revealed no solid core, but increasing the lower threshold to -250 HU revealed the solid component in the software.
"When we increase that lower threshold, the size of the lower solid core increases with it," he noted. The results showed the optimal HU threshold was -300 HU, which yielded a sensitivity of 90% (p = 0.89) with specificity of 88%.
| Differentiation of solid component with different lower thresholds | |||||||||
| HU | TP (n) | FP (n) | FN (n) | TN (n) | Sensitivity | Specificity | PPV | NPV | Total (n) |
| -500 | 82 | 14 | 1 | 10 | 99% | 42% | 85% | 91 | 107 |
| -450 | 80 | 8 | 3 | 16 | 96% | 67% | 91% | 94 | 107 |
| -400 | 78 | 7 | 5 | 17 | 94% | 71% | 92% | 87 | 107 |
| -350 | 76 | 4 | 7 | 20 | 92% | 83% | 95% | 74 | 107 |
| -300 | 75 | 3 | 8 | 21 | 90% | 88% | 96% | 72 | 107 |
| -250 | 70 | 3 | 13 | 21 | 84% | 88% | 96% | 62 | 107 |
| -200 | 64 | 3 | 19 | 21 | 77% | 88% | 96% | 53 | 107 |
| -160 | 54 | 4 | 29 | 20 | 65% | 83% | 93% | 42 | 107 |
| Manual | 51 | 1 | 32 | 23 | 61% | 96% | 98% | 42 | 107 |
TN = true negative; TP = True Positive; FN = False Negative; TN = True Negative; PPV = Positive Predictive Value; NPV = Negative Predictive Value.
For results, the optimal setting turned out to be -300 HU.
Another study question asked if the solid component of a part-solid lesion could be measured accurately in the same automatic segmentation.
"With our automatic measurements the mean size of the solid component showed an almost perfect linear relationship with a chosen lower threshold of the segmentation," Scholten said.
At a threshold of -130 HU, semiautomatic measurements of the diameter of the solid component (mean 2.4 mm, SD 2.7 mm) were comparable to manual measurements at mediastinal window setting (mean 2.3 mm, SD 2.5 mm [p = 0.63]). The Fleischner Society recommends use of a mediastinal window, which seems to make the solid core appear significantly larger, he added.
Semiautomatic segmentation of subsolid nodules can differentiate between nonsolid and part-solid nodules with good sensitivity and specificity as long as no vessel segments are included in the segmentation. As for size, diameter of the solid component of the solid nodule as defined by the recommendations of the Fleischner society, can be measured automatically as well, which is comparable with manual measurements.
As long as no vessel segments are included in the segmentation, and the solid component of the subsolid nodule as defined by Fleischner Society recommendations can be measured automatically -- that is, as long as the performance of semiautomated segmentation in these nodules is comparable with manual measurements, Scholten concluded.