A new test can suss out the particular genetic signature of higher risk for prostate cancer in some individuals, according to a study presented Saturday at the conference of the European Society for Radiotherapy and Oncology (ESTRO33) in Vienna.
Surgery and precision radiotherapy are key to controlling prostate cancer, but the disease will return in up to one-half of patients due to spread of the disease outside the prostate gland, according to presenter Dr. Robert Bristow and colleagues at Princess Margaret Cancer Center. Bristow is also a professor at the University of Toronto, Canada.
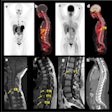
A new genetic test can make out the DNA-based "signature" that predicts treatment failure in patients undergoing radiotherapy or surgery, the study team reports. The tumor's genetic characteristics and its microenvironment were analyzed from biopsy tissue taken before treatment began.
The test predicts with nearly 80% accuracy which men are at high or low risk of prostate cancer recurrence, according to Bristow. It will be validated over the next few years to ensure its effectiveness in hospitals worldwide, according to the study team. With luck, the test will be able to determine which prostate cancer patients can be treated with local treatment, and which will need extra treatment, Bristow said in a statement accompanying release of the study.
The study is based on DNA analysis from biopsied tissue from 126 men considered to be at intermediate risk of recurrence. They were treated with image-guided radiotherapy (IGRT), which focuses the radiation on the tumor, and followed for an average 7.8 years.
To create the test, the study team used a process for analyzing tumor DNA called array comparative genomic hybridization (aCGH), which looks for missing, extra, or irregular sections of a patient's genome.