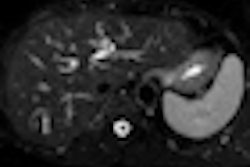
Patients with unresectable colorectal liver metastases who receive radiofrequency ablation (RFA) with chemotherapy treatment have longer progression-free survival than patients treated with chemotherapy alone, according to a new study by Dutch researchers.
Dr. Theo Ruers, head of the division of surgical oncology at the Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital in Amsterdam, presented the interim results of a phase II trial at the Society of Surgical Oncology's (SSO) annual cancer symposium, held earlier this spring in Phoenix.
The trial's objectives were to evaluate the safety and efficacy of this still-experimental treatment, with the primary end point measuring overall and progression-free survival 30 months after treatment completion. Hospitals in France, Germany, the Netherlands, and the U.K. participated.
Eligible patients had between one and 10 unresectable tumor lesions, each not exceeding 4 cm in diameter, some or all of which could not be completely resected. Patients who had extrahepatic disease were excluded. Eligibility did not preclude prior chemotherapy treatments.
Researchers enrolled 119 patients between April 16, 2002, and June 20, 2007. The trial was closed before reaching its goal of 152 patients; this was attributed to the fact that in the most recent years of enrollment, some of the participating oncology centers felt that the combined treatment, albeit experimental, was superior to chemotherapy alone. As a result, they were reluctant to enroll patients in the chemotherapy-only group.
Patients were randomly assigned to treatment groups. One cohort received only chemotherapy for six months. The other group received RFA and chemotherapy for six months, with or without resection of resectable lesions. This group received RFA treatment performed during laparotomy (51 patients), during laparoscopy (one patient), or percutaneously (four patients), with the others unspecified.
Patient characteristics
|
Complications were low for both groups, according to Ruers, considering the fragility and health of the patients. One patient died after RFA and resection, three patients had cardiac failure or infarction, and 10 patients required hospitalization for an average of five days.
Patients who received the combined treatment had a median progression-free survival of 16.8 months. By comparison, patients who received only chemotherapy had progression-free survival for 10 months.
If local recurrence in the patients who received the combined treatment was calculated only for the liver lesions that had been treated, the local recurrence rate would drop to 6.5%, according to Ruers. The majority of disease recurrence was in new tumor lesions in the liver.
At the end of 12 months, 20% more of the patients who received the combined treatment were alive, compared with patients who received chemotherapy alone. Only 39.4% survived in this latter group, compared with 60% of the patients who received the combined treatment.
By Cynthia E. Keen
AuntMinnie.com staff writer
June 22, 2009
Related Reading
PET scans facilitates decision-making for colorectal metastases in liver, January 10, 2007
Imaging unreliable for detection of colorectal liver metastases, September 6, 2006
RF ablation for genitourinary carcinoma aces strict cost-effectiveness criteria, July 31, 2001
Prior chemotherapy doesn't affect outcomes in liver tumor ablation, January 24, 2001
Copyright © 2009 AuntMinnie.com