A new study of pediatric MRI safety in the brain presented at the 98th German Radiology Congress (Deutscher Röntgenkongress, RöKo 2017) has added to the mounting evidence supporting the superior safety of macrocyclic gadolinium-based contrast agents (GBCAs) when compared with linear MRI contrast.
Dr. Dirk Klee, a pediatric radiologist at Heinrich Heine Medical Faculty, University Hospital of Düsseldorf, explained that for the past 10 years, his institute, along with other hospitals in Germany, has used macrocyclic agents for all pediatric MRI. Speaking to AuntMinnieEurope.com after the congress, which finished in Leipzig on 27 May, he admitted this may not be the case across Europe and the U.S.
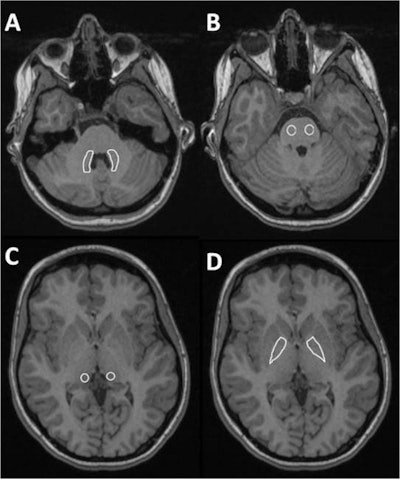 Sample images of four of the measured regions: A = dentate nucleus, B = pons, C = pulvinar, D = globus pallidus. All images courtesy of Dr. Dirk Klee and the German Radiological Society.
Sample images of four of the measured regions: A = dentate nucleus, B = pons, C = pulvinar, D = globus pallidus. All images courtesy of Dr. Dirk Klee and the German Radiological Society."Children have a whole lifetime ahead of them and we don't know what the potential toxic effects of gadolinium will be 30 or more years down the line," he said. "If a child had to undergo MRI with contrast to obtain a diagnosis, I would choose a macrocyclic agent that generated no visible changes after repetitive application on 1.5-tesla MRI, rather than a linear agent where significant signal intensity changes can be seen after repetitive application."
The global radiology community is one step closer to officially demonstrating that macrocyclic agents may be safer for children than linear agents, but further research is necessary, added Klee, who led the project.
The results of the study, due to be published by Radiology in an upcoming edition, were presented by Klee's colleague Christin Rademacher on 26 May at the RöKo.
Rademacher undertook the survey of MRI contrast agent deposits in children as part of her doctoral thesis and was awarded the German Pediatric Radiology Association's 2016 science prize. Her group in Düsseldorf looked at over 8,000 head MRIs, and identified 24 children who had received at least nine examinations using macrocyclic GBCAs (gadoteridol and gadoterate meglumine). The group found no evidence the contrast medium accumulates in the nervous system.
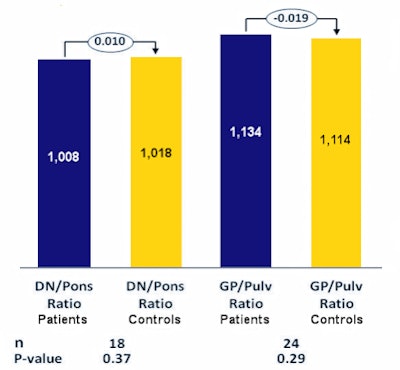 Median signal intensity ratios of patients (patients) and control group (control) between dentate nucleus/pons and globus pallidus/pulvinar. A paired t-test shows no significant difference between the two groups. DN = dentate nucleus; pulv = pulvinar; GP = globus pallidus; Ratio = ratio; n = number of patients.
Median signal intensity ratios of patients (patients) and control group (control) between dentate nucleus/pons and globus pallidus/pulvinar. A paired t-test shows no significant difference between the two groups. DN = dentate nucleus; pulv = pulvinar; GP = globus pallidus; Ratio = ratio; n = number of patients.To rule out age-related brain alterations, each child was assigned a comparator of the same age with no significant findings from a noncontrast head MRI. The signal intensities were then compared.
"We found the intensity was no higher than the control group in two relevant regions of the brain: the globus pallidus and dentate nucleus. And there was no correlation between number of MRIs and signal intensity," Klee noted in a statement released by the organizers of the RöKo.
The researchers interpreted this as evidence that macrocyclic GBCAs did not show significant accumulation in children's nerve tissue, and were safe even when administered repeatedly.
Key studies from Europe
The RöKo statement also pointed to recent studies that have found deposits in the nerve tissue of patients who have undergone repeated MRIs using linear contrast agents. While there was as yet no proof that nerve-tissue deposits of gadolinium contrast media have any negative clinical consequences for patients, they couldn't be ruled out, and the situation therefore needed to be monitored, according to Klee.
Other important research on the topic has come from Dr. Alexander Radbruch from University Hospital of Heidelberg and Dr. Diane Renz from University Hospital of Jena, as well as investigators from Rome, he added.
 Dr. Dirk Klee from Düsseldorf.
Dr. Dirk Klee from Düsseldorf."Although there has been other research about pediatric MRI contrast agents, we believe this is the first study in children's brains using an age- and sex-matched control group, and a case group with a 1.5-tesla MR scanner -- and the first that shows there are no changes visible in an MRI of the brain after administration of macrocyclic contrast agents," he noted.
In March 2017, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) recommended suspending the marketing authorization of four linear GBCAs because they cause a greater retention of gadolinium in the brain compared with macrocyclic GBCAs. The recommendation is subject to an appeal, which will be further reviewed by the PRAC and subsequently by the EMA's Committee for Medicinal Products for Human Use.
In May, the U.S. Food and Drug Administration (FDA) announced it would continue to assess the safety of GBCAs and would hold a public meeting in the future, but also noted it had not identified adverse health effects from gadolinium retained in the brain after the use of GBCAs. While the use of macrocyclic agents has been advocated by the U.S. National Institute of Health and the EMA, a final, legally binding decision has yet to be reached and the debate continues over whether macrocyclics should be used in preference to linear contrast agents.