Intravenous gadoteric acid is a safe and effective contrast agent for use in MR mammography, researchers from Germany have found. The study in the European Journal of Radiology is refreshing because it focuses on daily practice conditions.
The study included more than 1,500 patients, and each patient underwent high-resolution MR mammography. The research team, led by Drs. Tim Seithe and Joachim Braun from the radiology department at the Charité in Berlin, used a standardized questionnaire to report data, including patient demographics and medical history, characteristics of the MRI examination, and results in terms of diagnosis and safety for the patient.
"This noninterventional surveillance study with more than 1,500 examinations shows intravenous gadoteric acid [Dotarem, Guerbet] to be a contrast agent with sound diagnostic effectiveness and safety for use in MR mammography," they wrote (EJR, 14 October 2016). "Adverse events are rare and image quality is good to excellent."
Why study gadoteric acid?
Several intravenous gadolinium-based contrast agents are in clinical use, including gadoteric acid, which has been approved for use in brain and spine imaging and also whole-body MRI, including gastrointestinal, renal, urogenital, cardiac, breast exams, and bone and joint imaging in both children and adults, the authors noted.
However, the possibility remains of contrast-agent-related adverse effects, and not much research has been conducted -- specifically, a multicenter postmarketing surveillance study to generate and analyze data on diagnostic effectiveness and safety of intravenous gadoteric acid in MR mammography under daily practice conditions. Seithe, Braun, and colleagues sought to remedy this.
Large-scale multicenter study
The noninterventional surveillance study included 1,537 patients who underwent MR mammography with gadoteric acid at 15 centers across Germany. The researchers recorded the gender of 1,494 of the 1,537 patients, finding almost all (99.8%) were women and three were men.
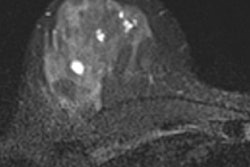
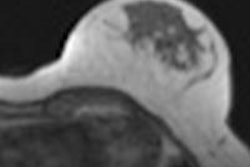
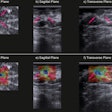
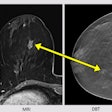
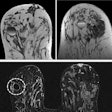
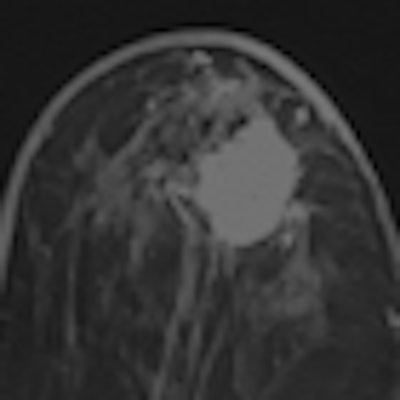
A diagnosis was established in 99.2% of all patients. In 91.6% of the patients, image quality was excellent or good. Histopathological exams were performed for 232 of 1,537 patients (15.1%) with invasive ductal carcinoma being the most frequent diagnosis. Using histopathology as the reference standard, intravenous gadoteric-acid-enhanced MR mammography confirmed the diagnosis in 93.5% of the patients.
Adverse drug reactions (tachycardia, dysphagia, urticaria, rash) occurred in five of 1,537 patients (0.3%), with one case classified as serious. All patients with adverse drug reactions fully recovered after the examination.
The percentage is comparable with what has been observed for various gadolinium-based contrast agents in other large postmarketing surveillance studies: 0.34% adverse event rate in the general population and patients with risk factors, 1.2% adverse drug reactions in a study on tolerance and clinical safety of gadobenate dimeglumine, and 2.4% in a study on clinical safety of gadopentetate dimeglumine, the researchers noted.
Safe and effective for MR mammography
The study has some limitations, however. It didn't include a control group, and risk and adverse drug reaction assessment may be subject to intersite bias in the participating study centers. Also, evaluation of image quality and accuracy are affected by the investigators' subjective impressions, the authors added.
"However, the effect is likely balanced by the large number of participating centers, and our data can be assumed to be fairly representative of the clinical practice," they wrote.
Also, the questionnaire allows for a large amount of data and is thus likely to identify very rare events, which was the case in this study.
"While we may conclude that adverse drug reactions and serious adverse drug reactions are very rare, it hence remains challenging to present a substantiated valid rate of serious adverse events because of its very low incidence," they noted. "Also, adverse events, which may have occurred at a later stage (e.g., later than 24 hours after gadoteric acid administration and the MRI examination) might have been missed."
Nonetheless, intravenous gadoteric acid is a contrast agent with sound diagnostic effectiveness and safety for use in MR mammography, the authors concluded.