Conventional cancer treatments using chemotherapy drugs can suffer from nonspecificity. New research shows MRI can direct cancer-killing cells to target sites. Not only does this technology confer the advantage of targeted treatment of cancer cells, it also enables anticancer agents to reach tumor sites in deep tissues that are normally inaccessible.
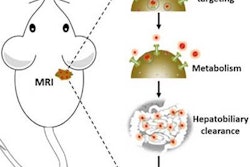
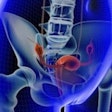
The technique, known as magnetic resonance targeting (MRT), uses the pulsed magnetic field gradients generated by an MRI scanner to guide cells containing ferromagnetic particles to target sites. The researchers, led by Munitta Muthana, PhD, at the University of Sheffield, U.K., showed that MRI scanners can noninvasively direct immune cells, which have been loaded with superparamagnetic iron oxide nanoparticles (SPIOs) and tumor-killing virus, to specific tumor regions in mice. They found that MRT enhanced the delivery and uptake of the magnetic cancer-killing cells into both primary prostate tumor and metastatic tumor site in lungs, leading to improved therapeutic outcome (Nature Communications, 18 August 2015, Vol. 6:8009).
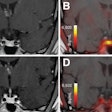
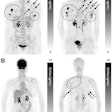
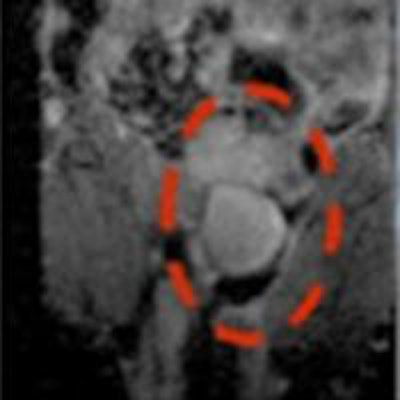
 Using MR scanners to steer cancer-killing cells to tumors in mice significantly delayed tumor growth compared with a control group.
Using MR scanners to steer cancer-killing cells to tumors in mice significantly delayed tumor growth compared with a control group."This is the first time whereby MRT, using an MRI scanner, has been used to target cell-based anticancer therapies to tumors located in inaccessible sites," Muthana said. "This not only increased the therapeutic payload at the target site whilst avoiding delivery to healthy tissues, but also led to tumor shrinkage and a significant reduction in metastasis."
Targeting tumor cells
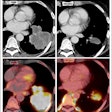
Before applying MRT in mice, Muthana and colleagues first tested the feasibility of a preclinical 7-tesla MRI system in vitro. They wanted to find out whether this could generate enough force to drive SPIO-loaded immune cells across an in vitro model of blood vessel cells, toward the direction of a 3D culture of tumor cells. The researchers used only 50% of the maximum magnetic strength of the preclinical MRI system, at 300 mT/m, to mimic the capability of clinical scanners. Uptake of immune cells guided by MRT into the tumor culture was 10 times higher than controls, which were exposed to a static magnetic field without a pulsed gradient.
Having shown that MRT works in vitro, the researchers next evaluated the MRT technology in mice. They injected magnetic immune cells into mice harboring primary prostate cancer, with metastatic tumors in the lungs. MRT exposure enhanced uptake of magnetic immune cells six-fold in primary prostate tumors, and more than four-fold in lung metastases; in comparison to controls without MRT guidance. In addition, MRT effectively distributed the magnetic immune cells throughout the tumors, with minimal cell clumping in the tumor blood vessels. This is in contrast to control experiments, where SPIO-labeled immune cells showed difficulty penetrating into the tumor center.
Arming the SPIO-labeled immune cells with the tumor-killing virus Seprehvir, the researchers administered the killer cocktail into the tumor-harboring mice. Mice subjected to MRT showed significant reduction in primary tumor growth and number of metastases. The team also showed that cell death in the primary tumor occurred to a greater extent in MRT-exposed mice than in the control group.
Promising clinical potential
The beauty of this technology lies in its potential applications not only in tumor targeting, but also in other cell-based therapies that require delivery of agents to specific sites. "This could be used to target drugs or nanodevices to organs or tissues that are difficult to access. A good example here would be the brain," Muthana explained.
Muthana notes that challenges remain in translating this technology for clinical application, warranting further studies into optimization of the MRT exposure protocol. "We would like to perform more experiments looking at timing, to see if we can reduce the length of time a subject would be in the scanner, and still achieve the same therapeutic effect," she explained. "This would reduce any discomfort from prolonged confinement and exposure to the scanner. Also, we would like to perform some mathematical modeling to scale this up, and make predictions about possible use in humans."
Nonetheless, Muthana is optimistic about translating the technology into clinical settings in the future. "Given that the scanners are already available in most clinics, and the virus we used has already been manufactured to clinical grade, we could predict to see a human clinical trial in the next three to five years," she told medicalphysicsweb.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.