Evaluation of treated hepatic lesions is challenging, so it's vital to adapt the imaging strategy and evaluation criteria according to the treatment used and underlying tumor, noted award-winning French researchers at last week's European Society of Gastrointestinal and Abdominal Radiology (ESGAR) annual meeting in Salzburg, Austria.
It is important to become familiar with the different treatment modalities available for primary and secondary liver lesions, and know the most suitable imaging modality to evaluate tumor response, stated Dr. Carmela Garcia Alba and colleagues from the department of radiology at Hôpital Saint Antoine in Paris, whose e-poster received a certificate of merit. They also said it's important to understand treatment-induced changes in the lesion and the surrounding parenchyma as well as its correlation with imaging findings.
Radiologists must also acknowledge the limits of the Response Evaluation Criteria In Solid Tumors (RECIST), as well as recognize the usefulness of modified criteria for hepatocellular carcinoma (HCC; mRECIST) and gastrointestinal stromal tumors (GIST; Choi), plus the need for a semiologic analysis of tumor composition after treatment, they emphasized.
Imaging plays a major role in oncology, not only at the initial phase of diagnosis and staging of neoplastic conditions, but also in the therapeutic phase, when imaging is crucial for treatment evaluation and eventual change of strategy/agent. Traditionally, this imaging evaluation of response is done by means of morphologic imaging, focusing on tumor size. The introduction and development of new agents and interventional procedures renders the evaluation of tumor response more and more complex.
"It is important to understand the mechanism of action of the different treatments to understand changes induced in liver lesions and correctly interpret tumor response," the authors stated. "Some new agents inducing devascularization and necrosis have little effect on tumor size and response criteria have been modified according to that."
Based on the type of treatment used and the lesion being treated, it is necessary to adapt the imaging technique to better identify and understand induced changes in the tumors, and this makes it possible to correctly implement the adequate response criteria that best suits the situation, they continued.
The four main treatments are as follows: classic systemic chemotherapy, targeted therapies/antiangiogenic agents, intra-arterial therapies, and thermal ablation.
Classic systemic chemotherapy
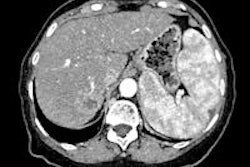
This is mainly used for metastatic disease. The most frequent primary tumors are colorectal cancer, breast, and lung. Their efficacy is based on their cytotoxic action, implying citoreduction and apoptosis. This is represented, on imaging, as a decrease in tumor size, and less importantly, a decrease of tumor enhancement. The imaging protocol most suited for the evaluation of classic chemotherapy is portal phase multidetector CT (MDCT). With an 80-90 second delay, it is possible to achieve the highest attenuation of the liver parenchyma and thus a good liver to lesion conspicuity.
Use the RECIST 1.1 criteria, the 1D response criteria for solid tumors last updated in 2009, the authors recommended. These criteria include a maximum of five target lesions (with a maximum of two per organ). For each, it's necessary to measure the longest diameter in the axial plane. It uses the sum of diameters (of the target lesions) as the central thread of objective tumor regression or progression. This is then a morphologic quantitative analysis. Partial response is defined as the decrease of at least 30% of the sum of diameters, with no new lesion or progression of nontarget lesions.
Targeted therapies/antiangiogenic agents
These treatments are mainly intended for hypervascular tumors. Their main mechanism of action is angiogenesis inhibition, thus inducing tumor ischemia and necrosis, sometimes hemorrhagic. This is translated, in imaging, as a decrease in tumor enhancement. There may be a paradoxical increase in tumor size due to the necrosis. Hemorrhagic changes will show as hyperdense on CT or hyperintense on T1-weighted imaging. The aim of tumor response evaluation is to evaluate the portion of vascularized, viable tumor.
Intra-arterial therapies
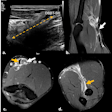
Based on the difference between blood supply between HCC (100% arterial origin) and normal liver (70% portal and 30% arterial), a high dose of drugs can be delivered to these tumors via the hepatic artery with little liver damage. These treatments include conventional transarterial chemoembolization (cTACE), drug-eluted bead transarterial chemoembolization (DEB-TACE), and radioembolization (RE Y90).
DEB-TACE uses particles (usually 300-500 µm) loaded with doxorubicin. There is no hyperdensity, so eventually follow-up can be done with MDCT, according to the researchers. As far as possible, the follow-up of HCC lesions should be done with MRI because its sensitivity and specificity are higher than that of CT.
In Europe, RE-Y90 is used mainly in clinical trials for intermediate stage HCC, particularly if the main portal vein or branch is invaded, and cholangiocellular carcinoma, liver-only disease. Its efficacy is based on the delivery of radioactivity through small caliber microspheres, without additional ischemic effect. Effects on tumor response can be evaluated only after six to nine months. Imaging evaluation can be difficult because liver fibrosis is induced in the treated lobe. Fibrosis will show as T2 hyperintensity, and this fibrosis must not be misinterpreted as tumor progression.
"Beware of radiation-induced fibrosis!" they warned.
Thermal ablation
The most common treatments for liver lesions are radiofrequency ablation (RFA) and microwave ablation (MW). They are considered a curative treatment of HCC nodules with a diameter of up to 3 cm, mainly if hepatic resection is not possible, or in cases of recurrent HCC after surgery. The treated lesion has spontaneous T1 hyperintensity, and subtracted images may help evaluate residual tumor enhancement. Remember the thin, homogeneous rim of granulation tissue, the authors noted. T2-weighted and diffusion-weighted imaging is useful for follow-up to depict local recurrence.
The vast majority of HCC are vascularized from the hepatic artery and its branches. Before chemoembolization, the hepatic arterial anatomy must be checked, and it is vital that its major variants are recognized on the pretreatment CT scan or arteriography, they emphasized. These tumors may acquire atypical "parasitic" vascularization from extra-hepatic arteries recruited by the tumor.
There are some recognized risk factors, including a past history of resection, hepatectomy, or repeated chemoembolization, and large peripheral, subcapsular, exophytic tumors. In addition, finding a large diameter extra-hepatic artery running toward the liver or a vascular pedicle belonging to the tumor itself on the pretreatment CT scan is evidence that the tumor has acquired "parasitic" vascular supply.
All of these findings should be noted on the CT or MRI report in the assessment of an HCC and must be taken into account by the interventional radiologist during chemoembolization.
Overall, choosing the right treatment is an increasingly complex issue, and there is a general move away from a "one size fits all" approach to personalized medicine. Imaging for evaluation has to adapt to this evolving concept, they concluded.