An online x-ray imaging system could provide 3D deformable motion tracking for use in scanned proton therapy, according to researchers at the Paul Scherrer Institute (PSI) in Villigen, Switzerland.
Organ motion can have an adverse impact on any dynamic radiotherapy delivery and is particularly problematic for spot scanned proton therapy. The magnetically deflected pencil beam used for spot scanning does, however, lend itself to the implementation of tumor tracking, considered the optimal method for mitigating motion. Unfortunately, there still remains the fundamental challenge of accurately determining target motion during treatment.
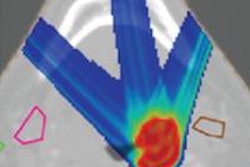
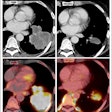
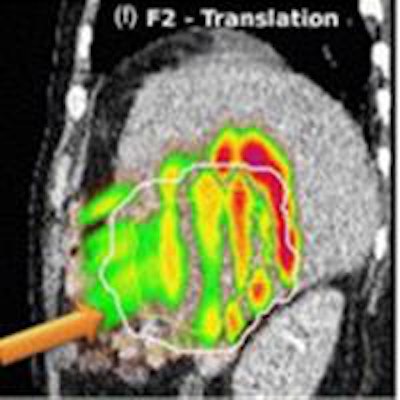
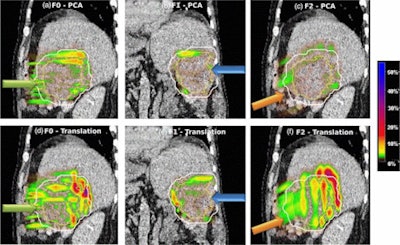 Absolute dose difference of 4D dose distributions considering ground truth motion versus reconstructed motion from either the PCA model (top) or the 2D translation model (bottom) prediction for three fields (the arrows indicate the field direction). All images courtesy of Ye Zhang.
Absolute dose difference of 4D dose distributions considering ground truth motion versus reconstructed motion from either the PCA model (top) or the 2D translation model (bottom) prediction for three fields (the arrows indicate the field direction). All images courtesy of Ye Zhang.At the PSI, the newest proton therapy gantry (Gantry2) is equipped with a beam's eye view (BEV) imaging system that can acquire 2D x-ray fluoroscopy images during proton therapy. But what's really needed is information regarding the 3D deformable motion throughout the whole target region. To achieve this, the PSI team has proposed a method for extracting 3D motion from surrogate motion detected using the BEV system (Physics in Medicine and Biology, 2013 November 21, Vol. 58:24, pp. 8621-8645).
"In many ways, pencil-beam scanning with particles is an ideal modality for tumor tracking," explained PSI researcher Ye Zhang. "However, this is currently limited by the lack of online, and accurate, dense 3D motion monitoring methods that can also predict the potential range changes resulting from anatomical motion. We hope that the methods introduced in this paper could be a potential solution to this problem."
The proposed method relies on pretreatment acquisition of a patient's motion data, recorded over a number of breathing cycles using 4D MRI. These data are employed to create patient-specific motion models using principle component analysis (PCA). The motion models can then be combined with BEV images of a surrogate (such as the diaphragm or fiducial markers) to determine the 3D target motion.
Simulated scenario
To study the feasibility of the proposed motion reconstruction scenario, Zhang and colleagues extracted motion data from 4D MRI liver studies of 11 volunteers, over 130 continuous breathing cycles (with 10 time steps for each cycle). They used the first 1,000 time steps to compute patient-specific motion models for each of the 11 subjects and the remaining 30 breathing cycles to generate simulated 4D CT datasets.
From these time-resolved CT images, the researchers generated digitally reconstructed radiographs (DRRs), using the geometric parameters of the BEV system and the tumor location with respect to the gantry isocenter. They then used tracking algorithms to extract the motion of the diaphragm or fiducial markers, as visualized in the DRRs. Finally, based on these tracked surrogate motions and the patient-specific motion models, they predicted the 3D deformable motion in the liver region for each subject.
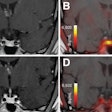
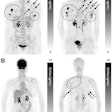
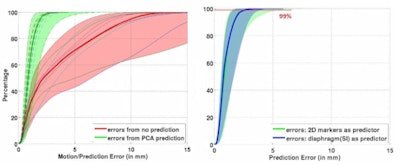 Left: PCA motion prediction using 2D fiducial markers. Right: PCA prediction using either tracked diaphragm SI-motion or 2D markers motion as predictor.
Left: PCA motion prediction using 2D fiducial markers. Right: PCA prediction using either tracked diaphragm SI-motion or 2D markers motion as predictor.The researchers examined the accuracy of their motion reconstruction by comparing the predicted results with the ground-truth motion (extracted directly from the 4D MRI data). Averaged over all 11 subjects, for 99% of predicted positions, median (maximum) errors were better than 2.63 (5.67) mm when tracking fiducial markers. Results were similar for tracking diaphragm motion, which resulted in a median (maximum) 99% prediction error of 2.7 (4.2) mm.
Dose differences
To further investigate the feasibility of motion tracking for scanned proton beam therapy, the PSI team examined the effect of motion prediction errors on the resulting 4D dose distributions. For all subjects, they calculated 4D dose distributions (for a single field and using Gantry2's scanning parameters) using both the ground-truth motion and predicted motion. Predictions were performed using both the PCA model, where full 3D motion is reconstructed, and a translation model, in which every point in the liver is assigned the same motion vector of the fiducials.
Comparing absolute dose differences between ground truth-based and prediction-based plans (using fiducial markers) showed that motion predictions from the translation-only scenario were poor, with maximum dose differences of more than 50%. Plans based on PCA-predicted motion, on the other hand, were similar to those based on ground-truth motion. Here, absolute dose differences of more than 5% occurred in only 3.61% (median) or 15.13% (maximum) of dose calculation points in the irradiated volume. Similar results were seen when tracking diaphragm motion.
 The PSI research group.
The PSI research group.The authors conclude that, in the liver region at least, deformable 3D motion can be accurately reconstructed from sparse surrogate motion acquired using the BEV imaging system, provided that a patient-specific PCA motion model can be constructed beforehand. The fact that diaphragm motion proved a good surrogate for predicting respiratory motion suggests that tumor tracking could be achieved without the need for invasive fiducial implants.
Based on the study results, the PSI team is currently working on two projects. One is investigating the possibility of online and near-real-time reconstruction of the delivered dose distribution, to enable image-guided scanned proton beam tracking. The other involves retrospective 4D dose calculation, using BEV tracking and patient-specific motion models to reconstruct motion on a fraction-by-fraction basis. This information will be combined with log files describing the delivery parameters of each pencil beam to reconstruct the actual 4D dose distribution delivered in each fraction.
"Such an approach could be an important quality assurance tool for 4D treatment validation and verification in the future," Zhang explained. "For instance, from these retrospective comparisons, the initial treatment plan could be adapted if necessary in the following fractions to deal with any unforeseen motion effects."
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.