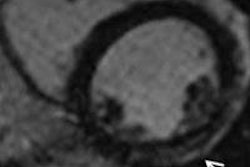
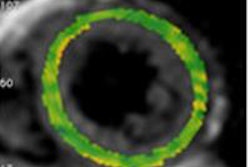
A study of patients with previous myocardial infraction (MI) undergoing late-gadolinium enhancement cardiac MR (LGE-CMR) found that scar length is strongly correlated to ventricular tachycardia cycle length (VTCL), according to a study in Europace.
The accurate prediction of VTCL could be a promising option for improving the postimplantation programming of implantable cardioverter-defibrillators (ICD) and reducing shocks, the authors wrote.
"In our study including patients with an old MI and ICDs for primary or secondary prevention, we demonstrated a strong positive correlation between VTCL and scar extent parameters identified by LGE-CMR (particularly the scar mass)," wrote Dr. Joachim Alexandre and colleagues from Centre Hospitalier Universitaire de Caen in France (Europace, 1 November 2013).
"Bearing in mind that this ... was a single-center and retrospective study with a small number of patients, assessment of scar extent prior to ICD implantation by LGE-CMR could be a promising and beneficial option to optimize ICD programming and decrease appropriate and inappropriate shock in patients presenting an old MI."
Patients who have had a myocardial infarction are at risk for re-entrant ventricular tachycardia (VT) because of scar tissue that can be accurately identified by late gadolinium enhancement cardiac MR, the authors explained. "Although the ability of LGE-CMR to predict sustained VT in implantable cardioverter-defibrillator (ICD) recipients has been well established, its use to predict monomorphic VT (sustained or not) cycle length (CL) and so, optimize ICD programming has never been investigated."
"Implantable cardioverter-defibrillators (ICDs) reduce the risk of post-MI sudden cardiac death (SCD), but this beneficial effect is conditional by an optimal programming with a constant challenge which is to avoid inappropriate therapies caused predominantly by supraventricular tachyarrhythmias (SVT)," the study team explained.
Postinfarct VT is usually due to re-entry into the infarct zone, and a recent canine study of re-entrant VT showed that VT cycle length and scar geometry were highly correlated, the authors noted. The study also showed that measuring VTCL before electrophysiology studies could guide ablation therapy by estimating scar size and geometry.
The ability of late-gadolinium-enhancement MR to predict sustained VT in implantable ICD recipients is well established, however it's use to predict monomorphic VC cycle length has never been studied, the authors wrote.
This study aimed to evaluate whether postimplantation VTCL is correlated to scar extent, and whether the analysis of scar characteristics can help optimize ICD programming in post-MI patients over the long term.
CMR was performed on 66 consecutive patients with coronary artery disease who had undergone late-gadolinium-enhancement CMR before primary or secondary preventive ICD implantation. Patients with a history of amiodarone use, which lengthens CL, were excluded. All patients were also examined by transthoracic echocardiography before ICD implantation, the authors wrote.
All exams were performed on a dedicated 1.5-tesla CMR scanner (GE Healthcare) using TR 50 ms; TE 1.7 ms; flip angle 558; slice thickness of 7 mm; matrix size 256 x 216; and field-of-view of 360-420 mm. The authors used vector ECG gating to acquire 20 images per cardiac cycle. Localizer images were followed by steady-state free precession cine images. Contrast-enhanced images were acquired 15 seconds after bolus injection of gadoterate meglumine (Dotarem 0.15 mmol/kg, Guerbet).
The study team assessed scar extent by measuring scar mass, percent scan, and transmural scar extent in patients who had experienced MI 8.7±2.5 years before CMR. The endpoint was the occurrence of monomorphic VC over a median follow-up of 31 months.
In all, 26 patients (53%) experienced VT and six patients (12%) died, while three patients (6%) presented with a VF requiring ICD therapy.
Results in 49 patients (mean age 61±11, 90% male) showed all scar extent parameters were significantly correlated with the study endpoint.
Of note, left ventricular ejection fraction was not associated with the study endpoint, the group reported. And patients who received ICD implantation as secondary preventive therapy showed a positive correlation between scar mass and initial VTCL (P = 0.043) and a positive correlation between scar mass and first recurrent VT (r = 0.62, P = 0.034).
Univariate regression analysis showed that scar mass had the highest correlation with VTCL (r = 0.671, P = 0.0002). Receiver-operating characteristic (ROC) curve showed that scar mass can predict VTCL (area under the curve 0.977, P < 0.0001).
"Our observational study showed (i) a positive correlation between scar extent variables (scar mass, percent scar, and transmural scar extent) and the VTCL; (ii) that scar mass may be potentially a reliable noninvasive tool to predict postimplantation VTCL," Alexandre and colleagues wrote, cautioning that the study's preliminary conclusions need to be confirmed in a larger prospective cohort.
Scar quantification failed to predict monomorphic VT occurrence that was recorded by ICD devices requiring a therapy or not, but this was not surprising because in the literature, scar quantification has been shown to predict sudden cardiac death, and prolonged VT requiring ICD therapy, but the correlation between scar extent and nonsustained VT is uncertain, the group wrote.
"The new insight highlighted in our study is that the scar mass presented a high correlation with VTCL ... and as depicted in the ROC curve ... can accurately predict VTCL," they wrote. For a cut-off value of scar mass at 17.6 g, there is 100% specificity and a 94.4% sensitivity of experiencing VTCL > 300 ms.
Based on these results, the scar mass details could be useful in optimizing postimplantation ICD programming postmyocardial infarction, particularly in primary prevention, they wrote.
For a scar mass of 17.6 g, in the study population, for example, "it would probably be unwise to program a VT zone of < 300 ms due to the low probability of having a slow VT and the risk of inappropriate shocks related to [supraventricular tachycardias] SVT. On the other hand, for a patient with a scar mass < 17.6 g, the study criteria are consistent with study suggestions to "program a slow VT zone with a prolonged delay in therapy and an ATP optimization programming to avoid excessive and premature appropriate shocks," they wrote.
The authors cited the main limitations of the study as its retrospective design and relatively small sample size, and also noted limitations based on the "assumption that scar extent parameters are a major determinant of VTCL," which holds true for large, homogeneous, and dense scars, but for several reasons may not apply to small infarct scars, they wrote.