Bayer has initiated a Phase III clinical development program called Quanti, which aims to explore the effectiveness and safety of gadoquatrane, the company's gadolinium-based contrast agent.
The company said that gadoquatrane is a "highly stable" MRI contrast agent that has high relaxivity and could lead to a lower gadolinium dose for patients.
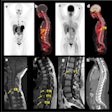
The Quanti program includes two Phase III studies, Quanti CNS and Quanti OBR, as well as a pediatric study. These studies are evaluating gadoquatrane at a dose of 0.04 mmol Gd/kg body weight. This represents a gadolinium dose reduced by 60 percent in contrast-enhanced MRI compared to standard dosing of established products.
Quanti CNS will investigate the efficacy and safety of gadoquatrane in adults with known or highly suspected pathologies of the central nervous system. Quanti OBR will explore gadoquatrane's performance in contrast-enhanced MRI in all other body regions, including the head and neck, thorax, abdomen, pelvis, and extremities. The pediatric study, meanwhile, will assess the pharmacokinetics and safety of gadoquatrane in children of all ages undergoing contrast-enhanced MRI.
In total, the Quanti program aims to enroll about 800 patients in 17 countries.